doi: 10.56294/hl2024.355
ORIGINAL
Children with autism spectrum disorder’s sleep patterns: a research analysis
Patrones de sueño en niño con trastornos del espectro autista: análisis de investigación
Bharat
Bhushan1 ![]() ,
Sameer Rastogi2
,
Sameer Rastogi2 ![]() ,
Vijay Jagdish Upadhye3
,
Vijay Jagdish Upadhye3 ![]() ,
Banani Jena4
,
Banani Jena4 ![]() ,
Anoop Dev5
,
Anoop Dev5 ![]() ,
Sunil Lawand6
,
Sunil Lawand6 ![]()
1Chitkara Centre for Research and Development, Chitkara University. Baddi, Himachal Pradesh, India.
2School of Pharmacy, Noida International University. Greater Noida, Uttar Pradesh, India.
3Parul Institute of Applied Sciences (PIAS), Parul University, Dept of Microbiology. Vadodara, Gujarat, India.
4IMS and SUM Hospital, Siksha ‘O’ Anusandhan (Deemed to be University), Department of Respiratory Medicine. Bhubaneswar, Odisha, India.
5Centre of Research Impact and Outcome, Chitkara University. Rajpura, Punjab, India.
6Krishna Institute of Medical Sciences, Krishna Vishwa Vidyapeeth “Deemed to be University”, Dept. of Pediatrics. Taluka-Karad, Dist-Satara, Maharashtra, India.
Cite as: Bhushan B, Rastogi S, Upadhye VJ, Jena B, Dev A, et al. Children with autism spectrum disorder’s sleep patterns: a research analysis. Health Leadership and Quality of Life. 2024; 3:.355. https://doi.org/10.56294/hl2024.355
Submitted: 01-03-2024 Revised: 13-07-2024 Accepted: 09-11-2024 Published: 10-11-2024
Editor:
PhD. Prof. Neela Satheesh
![]()
ABSTRACT
Sleep issues are common in young children with autism spectrum disorder (ASD), and although the Children’s Sleep Habits Questionnaire (CSHQ) is widely used for assessment, concerns about its ideal variable structure still exist. This research looked at the CSHQ’s variable structure in young children with ASD and explored the relationship between CSHQ variables and emotional, cognitive, and behavioral dysregulation. Children with ASD between the ages of 4 and 5 (n = 270) took part in the research. Two previously published CSHQ variable frameworks were tested for fit to the sample data using confirmatory factor analysis (CFA), and other frameworks were investigated using exploratory factor analysis (EFA). Regression analysis evaluated how differences in dysregulation symptoms were explained by the values of the Child Behavior Checklist variables. Previously published frameworks for children with ASD were not validated, a novel five-variable system discovered by EFA showed a great fit with the sample data. Although sleep characteristics were not generally linked to autistic symptoms, there was evidence of particular associations between them and aggression, stress, depression, and attention deficit problems. These results highlight how common sleep issues are in young children with ASD and indicate that the recently discovered CSHQ five-variable approach may be useful in future studies.
Keywords: Autism Spectrum Disorder (ASD); Kids Sleeping Disorder; Aggressive Attitude; Stress And Depression; Concentration Issues.
RESUMEN
Los problemas de sueño son comunes en los niños pequeños con trastorno del espectro autista (TEA), y aunque el Cuestionario de Hábitos de Sueño de los Niños (CSHQ) es ampliamente utilizado para la evaluación, todavía existen preocupaciones acerca de su estructura ideal de variables. Esta investigación analizó la estructura variable del CSHQ en niños pequeños con TEA y exploró la relación entre las variables del CSHQ y la desregulación emocional, cognitiva y conductual. Participaron en la investigación niños con TEA de entre 4 y 5 años (n = 270). Se comprobó el ajuste a los datos de la muestra de dos marcos de variables del CSHQpreviamente publicados mediante análisis factorial confirmatorio (AFC), y se investigaron otros marcos mediante análisis factorial exploratorio (AFE). El análisis de regresión evaluó cómo las diferencias en los síntomas de desregulación se explicaban por los valores de las variables del Child Behavior Checklist. Los marcos previamente publicados para niños con TEA no fueron validados, un novedoso sistema de cinco variables descubierto por EFA mostró un gran ajuste con los datos de la muestra. Aunque las características del sueño no estaban generalmente vinculadas a los síntomas autistas, había pruebas de asociaciones particulares entre ellas y la agresión, el estrés, la depresión y los problemas de déficit de atención. Estos resultados ponen de relieve lo comunes que son los problemas de sueño en los niños pequeños con TEA e indican que el enfoque de cinco variables del CSHQ descubierto recientemente puede ser útil en futuros estudios.
Palabras clave: Trastorno del Espectro Autista (TEA); Trastorno del Sueño Infantil; Actitud Agresiva; Estrés Y Depresión; Problemas De Concentración.
INTRODUCCIÓN
Sleep is an essential variable influencing kid’s development, behavior, and well-being. However, for kids diagnosed who have autism Spectrum Disorder (ASD), achieving sufficient and quality sleep can be more challenging compared to their typically developing counterparts.(1) Understanding the sleep patterns of kids with ASD not only sheds light on the unique struggles faced by these kids and their families but also opens up avenues for intervention and support that can enhance their overall quality of life. The neurological disorder known as autism spectrum disorder is marked by recurrent actions, limited passions, and trouble with social interaction affects approximately 1 in 54 kids in the United States. One of the most commonly reported co-occurring issues in ASD is sleep disturbances, with prevalence estimates ranging from 44 % to 86 %. These disturbances often include difficulty falling asleep, frequent night waking, reduced overall sleep time, and poor sleep quality.(2) While sleep issues are not a diagnostic criterion for ASD, they occur so frequently that their presence has become almost an expected part of the disorder’s presentation. The causes of these sleep issues in kids with ASD are complex and multifaceted. Some researchers have hypothesized that these disturbances might be due to biological variables inherent in ASD, such as genetic differences or abnormalities in melatonin regulation. Melatonin, a hormone that regulates the sleep-wake cycle, has been found in lower levels in individuals with ASD.(3)
On the other hand, environmental variables such as screen time, inconsistent bedtime routines, dietary issues, or sensory sensitivities common in ASD may also contribute to sleep disturbances. Moreover, stress and other co-occurring psychiatric conditions, which are often prevalent in individuals with ASD, can exacerbate sleep issues. Sleep disturbances in kids with ASD are more than just a night-time issue; they can also have significant implications for daytime behaviour and functioning.(4) Research has shown that sleep issues can exacerbate ASD symptoms, such as communal statement difficulties and monotonous performances. Kids with ASD and co-occurring sleep issues are also more likely to experience behavioural issues such as hyperactivity, aggression, and mood swings. These disturbances can negatively affect the child’s functioning at school and their ability to engage in social interactions. (5) In addition to impacting kids, these sleep issues can also create challenges for the families of kids with ASD. Caregivers often report high levels of stress and decreased quality of life due to their child’s sleep issues. This highlights the necessity of interventions targeting sleep disturbances to not only improve the child’s quality of life but also the well-being of the entire family.(6)
While sleep disturbances in kids with ASD are challenging, they are not insurmountable. Several evidence-based interventions have shown promise in improving sleep in these kids. These include behavioural interventions, such as creating a consistent bedtime routine and sleep environment, and pharmacological interventions, such as melatonin supplementation. However, the best intervention strategies often involve a combination of these approaches, individualized to suit the unique needs of each child.(7)
The CSHQ of kids who have ASD comprised an age range (4-5 years); however, by limiting our sample to nursery within a specific age range, we were able to prepare for behavioural and environmental alterations.
Part 2-related work, Part 3-methods, Part 4-results, and Part 5-conclusion with constraints and future scope compose the remaining sections of this paper.
Kids who have autism spectrum disorder are at a greater risk of developing sleep issues than other children because of fundamental biological and behavioural tendencies that make them predisposed to both extrinsic and intrinsic stressors that disrupt sleep. Calming techniques may be useful in enhancing sleep because there is mounting evidence that arousal dysregulation and sensory hyper-reactivity are connected with ASD.(8) Kids who have ASD experience sleep issues more frequently than kids who have TD, regardless of cultural variables. Despite the high reported incidence in their research, few parents actually sought help for sleep issues because of ignorance.(9) The primary diagnostic features of ASD also contribute to nighttime interruptions. Additional prospective studies are required to examine the effect of night-to-night changes on day-to-day changes in the intensity of ASD symptoms, as there is still insufficient evidence to support the opposite.(10) Sleep issues are substantially more common in kids who have autism spectrum disorders than in normally growing kids at all stages in China. Bedtime resistance, stress, delayed sleep begin, and tiredness during the day are the initial four. These sleep issues may be linked to their basic symptoms rather than the developmental stage of ASD. ASD clinic visits should routinely include a sleep evaluation, and during the intervention, education about good sleep hygiene is just as crucial as the management of biological causes.(11) Demonstrated the effectiveness of actigraphy when compared to polysomnography in assessing sleep quality metrics in a young group of kids who have ASD. Only when behavioural and sleep therapies have been unsuccessfully attempted can melatonin therapy be explored.(12) The long-term safety and distinctive efficacy profiles suggest that PedPRM has a considerable positive impact on insomnia in kids and teenagers who have ASD.(13) The cycle of sleep and wakefulness is an example of a circadian rhythm. Physiological or emotional cycles that repeat every 24 hours or so are known as circadian rhythms. A quantitative description of these rhythms would be “daily fluctuations of hormonal substances, core body temperature, rest-activity cycles, or transcription structures.” Circadian rhythms are thought to play a role in maintaining homeostasis, or homeostatic balance.(14) Sleep issues are prevalent in this demographic, according to a research in China that looked at nursery kids who have ASD and matched in age TD kids. The characteristics of the individual’s sleep patterns were also thoroughly examined, and they came to the conclusion that treatments aimed at addressing sleep issues would help Chinese nursery kids who have ASD’s emotional/behavioural issues and repetitive behaviours.(15)
METHOD
Participants Design and measurements
Dataset for this research were collected participants from a sizable multisite longitudinal research project that looked at the growth paths of a cohort of kids who have ASD that received a fresh diagnosis. The following criteria were satisfied for inclusion of the research’s kids
a. Were among the ages of 2 and 4 years and 11 months that’s when they were diagnosed;
b. Satisfied ASD criteria on the Autism Diagnostic Assessment Schedule, in the Social area and in a minimum one other area;
c. Satisfied the DSM-IV-TR criteria for ASD in the clinical opinion of doctors with diagnostic skills. Neuromotor issues, significant hearing or vision damage, and/or known genetic or chromosomal disorders were among the exclusion criteria.
In this research, we recruited all families who had kids who had completed the (CSHQ) while those kids were between the ages of 4 and 5. Our longitudinal research’s cross-sectional information was utilized. M-P-R are used to measure mental growth; the sample included 270 families presented in table 1.
|
Table 1. Samples of 270 families |
|
|
Sample Characteristics |
Value (SD) |
|
Number of Families |
270 |
|
Mean Age at Diagnosis (41,78 months) |
7,46 months |
|
Mean Age at CSHQ Completion |
52,9 months |
|
Male Kids (%) |
84,7 % |
|
Cognitive Development Assessment Availability (%) |
85,8 % |
|
Assessment Availability Description |
Available for 241 participants |
|
M-P-R (Sample) (68,44 months) |
30,79 |
|
M-P-R (Standard) |
Mean: 100, SD: 15 |
Serious autism symptom
ADOS is a semi-structured, standardized test where people participate in tasks meant to evaluate social and interpersonal behaviours suggestive of ASD symptoms. The examiner is trained to administer the test. The ADOS has great internal consistency, high test-retest dependability, and good internal dependability. Clinical researchers with expertise in dependability research conducted the ADOS. To enable comparisons between modules, calibrated intensity scores remained utilized for “Social Affect, Repetitive and Limited Behaviours, and Overall Score” to take into account differences in proficiency in languages and module administration. Higher scores indicate more severe ASD; values range from 1 to 10.
Child dysregulation symptoms
Kids’ stress feelings of depression, aggressive attitude, and concentration issues were evaluated using the CBC1,5-5 on a 3-point scale “(0-not true, 1-occasionally true, and 2-frequently true)”. There are 20 items on the Aggressive Attitude subscale. There are 14 items in the subscale for stress and depression. 11 items make up the Concentration Issues subscale. In a sample of nursery kids who have ASD, recent research supported the variable framework of the CBCL 1,5-5.
Related Symptoms for Sleep
The 33 items that make up the eight sections of the shortened CSHQ are: SDs, Sleep Stress, DS, BR, NW, Sleep-Onset Delay, Parasomnias, and SD. On a three-point scale, participants represent how often they typically engage in sleep behaviour during the most recent average week, presented in table 2.
|
Table 2. Sleep behavior |
|
|
Conditions |
per week |
|
Regularly |
6-7 times |
|
Occasionally |
3-5 times |
|
Exceptional |
0-2 times |
Higher scores indicate more sleep issues, and both the overall score and subscale scores are determined. According to reports, the sensitive clinical cutoff for identifying likely sleep issues is an overall CSHQ value of 41.
Data analysis plan
There were two stages involved in developing psychometric data to back up the summary of the CSHQ values as indicating sleep issues in kids who have ASD. First, we used CFA to look at the CSHQ system of variables in kids who have ASD. We prepared an EFA to look into other systems in the event that the first-stage CFAs did not discover a satisfactory fit in our data.The previously improved CSHQ four-variable solution, as well as the five-variable solutions, was subjected to a CFA to determine which offered a better fit to our test CSHQ information. We took into account the following cut-off values for the fit quality statistics while assessing the system’s fit: When the RMSEA is less than 0,05, the fit is good. The 90 % RMSEA confidence range was supplied. The =(TI> 0,95) and comparative fit index (CI> 0,90) indicate strong fit of the system. To provide a better understanding of the fit of the system, we used the The fit quality chi-square test which is affected by the number of samples. Mplus 7,4 performed CFA; if CFA results did not fulfill “good fit” standards, EFA was employed in the following step to determine a different variable framework.
If an EFA was chosen, all 33 items would be used to discover the best number of latent structural components. We chose the WLSMV estimate because the CHSQ has a 3-point item answer scale.This method uses ordinal item response information when the normality condition is exceeded. WLSMV adapts to lacking information. We used oblique Geonim rotations GR for ordinary items and variable correlation.The EFA started by determining how many variables would best represent the internal framework of CSHQ. The eigenvalues (EV) >1 “rule, scree plot, and Horn’s parallel analysis (PS)” helped us to decide. Random variables decide Horn’s PS’s variable retention.Next, we examined the RMSEA fit score for every variable solution with EV>1 supported by the PS. The EC and PS findings pointed to several variable solutions. Finally, we used our clinical and theoretical expertise to assess the simple-framework understanding of loadings variable following GR for systems with an appropriate fit based on RMSEA. A GR is advised for “variable architectures with statistically inconsequential, insignificant crossovers and a comprehensible structure matrix with relationships between the variables.”
To avoid over-conservatism, we eliminated only items with loadings below 0,35 on all criteria. To enhance understanding, objects must not load on multiple variables, and at least three items per variable should be supplied to ensure proper identification. After accounting for the statistical variables, feasible variable solutions were investigated based on component material and interesting structures. Each variable’s CA was evaluated. Mplus 7,4 performed CFA and EFA.
Relationship between autism and dysregulation symptoms
The determination of this research was to observe the connection between the severity of ASD symptoms and the CSHQ sub scales. In order to adjust for the abnormality of ordinary variables, Spearman’s rank order connections were calculated. Next, we investigated the relationship between child dysregulation behaviors and subscale scores on the revised CSHQ. So, using the CBCL as a measurement, we performed hierarchical regression analyses in order to look at predictors of symptoms linked to aggression, depression, stress, and concentration issues.
• Kid’s ages at the conclusion of the CSHQ and family demographic variables were entered as variables.
• Scores from the CSHQ subscale for kids were entered.
SPSS version 25 was used for bivariate correlation analysis and hierarchical multiple regression (HMR) system.
RESULTS
Descriptive statistics (DS)
DS should be emphasized that the reaction distributions for many items were extremely skewed. Both parasomnia and breathing disorders during sleep had relatively low endorsement frequencies. According to the accepted cut-off value of > 41, 66,9 % of the kids in the present sample were classified as “poor sleepers” based on their CSHQ total value.
Items of factor loadings for CSHQ GR
Item 3: Sleeps in their own bed, Item 5: Requires parental presence in the room to fall asleep, Item 8: Experiences fear when sleeping alone, Item 16: Transfers to another person’s bed, Item 1: Initiates sleep at a consistent time each night, Item 2: Achieves sleep within a 20-minute timeframe , Item 6: Faces difficulties during the bedtime routine, Item 9: Experiences insufficient sleep, Item 10: Achieves an appropriate amount of sleep, Item 11: Maintains a consistent sleep duration daily, Item 13: Engages in sleep talking, Item 15: Experiences sleepwalking episodes during the night, Item 22: Wakes up from sleep with episodes of screaming, Item 23: Wakes up feeling alarmed or frightened by a dream, Item 24: Wakes up once during the nighttime, Item 25: Experiences multiple awakenings during the nighttime, Item 18: Produces loud snoring sounds during sleep, Item 19: Exhibits episodes of sleep apnea, where breathing temporarily stops, Item 20: Makes snorting or gasping sounds during sleep, Item 29: Faces challenges in getting out of bed, Item 26: Awakens independently without external assistance, Item 28: The child is awakened by adults or siblings, and Item 30: Requires an extended period to fully wake up and become alert.
The Supplementary contains the DS for each of the CSHQ elements presented in Table 3. In Table 3, Factor 1, 2, 3, 4, and 5 denotes MW, SD, SDs, NW, and STR.
|
Table 3. CSHQ GR Factor Loadings with five factors |
|||||
|
Item # |
Factor 1 |
Factor 2 |
Factor 3 |
Factor 4 |
Factor 5 |
|
5 |
0,89 |
-0,09 |
0,11 |
-0,03 |
0,02 |
|
16 |
0,51 |
0,11 |
0,32 |
0,14 |
-0,23 |
|
2 |
-0,08 |
0,49 |
0,36 |
-0,45 |
0,32 |
|
9 |
-0,11 |
0,74 |
0,25 |
0,08 |
-0,03 |
|
11 |
0,14 |
0,71 |
0,12 |
0,08 |
0,25 |
|
15 |
-0,11 |
0,09 |
0,45 |
0,22 |
-0,05 |
|
23 |
0,05 |
-0,09 |
0,84 |
0,07 |
0 |
|
25 |
0,11 |
0,11 |
0,61 |
0,18 |
-0,26 |
|
19 |
-0,17 |
0,31 |
0,07 |
0,59 |
0,07 |
|
29 |
-0,03 |
0,08 |
0,21 |
0,12 |
0,78 |
|
28 |
-0,07 |
-0,11 |
0,05 |
0,32 |
0,86 |
|
3 |
0,74 |
0,22 |
-0,18 |
0,05 |
0 |
|
8 |
0,92 |
-0,05 |
0,05 |
-0,05 |
0,08 |
|
1 |
0,26 |
0,59 |
-0,08 |
-0,08 |
0,34 |
|
6 |
0,09 |
0,37 |
0,29 |
-0,06 |
0,32 |
|
10 |
0,04 |
0,84 |
-0,05 |
0,21 |
-0,14 |
|
13 |
0,06 |
-0,07 |
0,52 |
0,32 |
0,07 |
|
22 |
-0,08 |
-0,09 |
0,88 |
-0,11 |
0,08 |
|
24 |
0,31 |
0,08 |
0,62 |
0,05 |
-0,27 |
|
18 |
0 |
-0,04 |
-0,04 |
0,79 |
0,19 |
|
20 |
0,02 |
-0,02 |
0,12 |
0,92 |
-0,07 |
|
30 |
0,11 |
0,03 |
0,32 |
−0,05 |
−0,05 |
|
26 |
0,15 |
0,08 |
−0,05 |
0,31 |
0,92 |
CFA and EFA
The 27 CSHQ components included five-variable solution were evaluated for fit indices, and the results showed moderate to poor system fit to the data sample, RMSEA = 0,06; x2 (319) = 698,80, p < 0,001; CI = 0,65 and TI = 0,68. The four-variable solution, which included 23 CSHQ items, had its fit indices evaluated, and the results showed poor to moderate fit of the system to the data sample, RMSEA = 0,05; x2 (218) = 565,66, p < 0,0001; CI = 0,74 and TI=0,69.
The absolute fit represents (RMSEA) was at an acceptable limit, and the values generated for the comparison fit statistics suggested that neither CFA had a good system fit. As a result, it was appropriate for us to consider how the CSHQ assessment system might be enhanced. Our model of 4 to 5-year-olds kids who have ASD was used to investigate the variable framework; we performed an EFA.
Eight variables with EV >1,00 developed in the first stage of the EFA, accounting for a total of 56 % of the total variation.
Good fit was found when looking at the RMSEA acceptable measurement for the five-variable option like (“MSEA = 0,029; x2 (141) = 253,25*, p < 0,0001; CI = 0,87 and TI = 0,79). Eight items had loadings that were < 0,35 for the five-variable solution, and two items had cross-loading on several variables, according to an examination of the item loadings. So, these things were taken away to see if the answer was stable, we ran the EFA again.
Our findings validated the five-variable system after the 10 elements were removed. The final system has good fit indices (RMSEA = 0,04; x2 (152) = 252,18, p < 0,001; CI = 0,87; TI = 0,86); Table 3 displays the composite score for each element also includes the CA and DS.
Associations with ASD symptoms intensity
There was often no correlation between CSHQ subscale results and the intensity of autism symptoms as measured by ADOS Communal Affect, Limited and RepetitivePerformances, and the Entire measured intensity values mentioned in Table 5. Table 4 denotes the Correlation of Spearman’s CSHQ Subscales items. The only correlation was between the intensity value for Restricted and Repeated Behaviors and Sleep Disordered Breathing. Figure 1 depicts the correlation of mean (M) and standard deviation (SD) for the CSHQ Subscales.
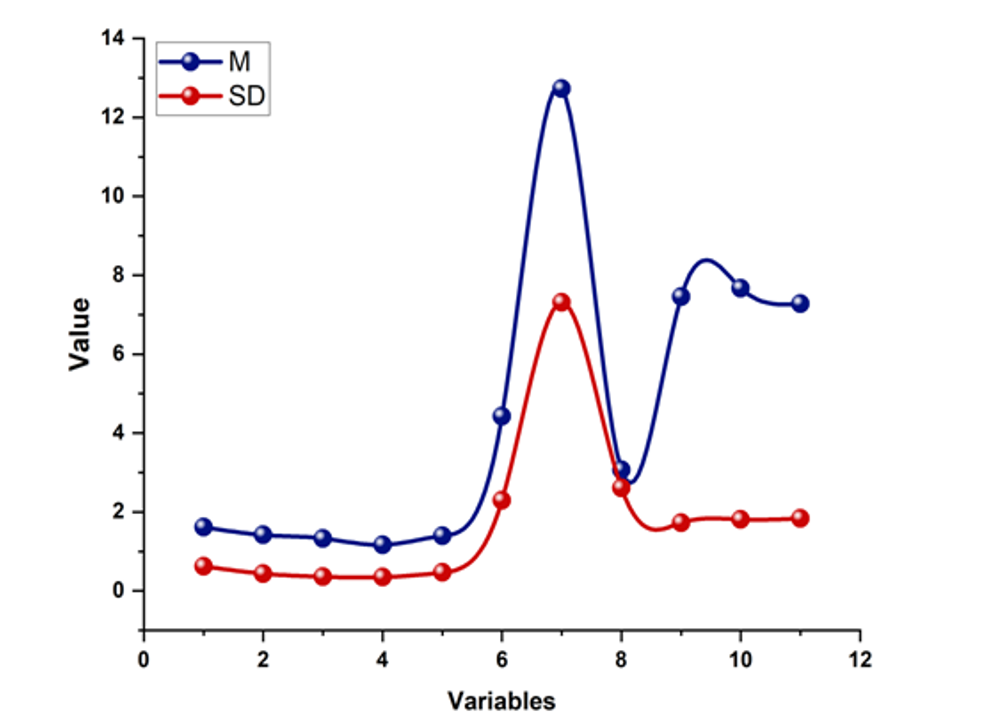
Figure 1. M and SD for Spearman’s CSHQ Subscales
|
Table 4. Items of Correlation of Spearman’s for the CSHQ Subscales |
|
|
Items |
Variables |
|
1 |
STR |
|
2 |
SD |
|
3 |
NW |
|
4 |
SDs |
|
5 |
MW |
|
6 |
Concentration issues |
|
7 |
Aggressive attitude |
|
8 |
Stress and depression |
|
9 |
ASD overall intensity |
|
10 |
RRB intensity |
|
11 |
Social affect intensity |
|
Table 5. Correlation of Spearman’s for the CSHQ Subscales |
|||||||||||
|
Variables |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
|
1 |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
2 |
0,29** |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
3 |
0,38** |
0,42** |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
|
4 |
0,17* |
0,14 |
0,17** |
1 |
- |
- |
- |
- |
- |
- |
- |
|
5 |
0,13* |
0,31** |
0,19* |
0,24** |
1 |
- |
- |
- |
- |
- |
- |
|
6 |
0,13* |
0,32** |
0,18** |
0,18** |
0,18** |
1 |
- |
- |
- |
- |
- |
|
7 |
0,12* |
0,41** |
0,33** |
0,12 |
0,17** |
0,53** |
1 |
- |
- |
- |
- |
|
8 |
0,29** |
0,32** |
0,18** |
0,17* |
0,34** |
0,28** |
0,51** |
1 |
- |
- |
- |
|
9 |
0 |
-0,11 |
-0,04 |
-0,12 |
-0,07 |
0 |
-0,15 |
-0,08 |
1 |
- |
- |
|
10 |
0,07 |
0,04 |
0,09 |
-0,17** |
-0,05 |
0,04 |
-0,08 |
0,04 |
0,56** |
1 |
- |
|
11 |
-0,04 |
-0,08 |
-0,08 |
0 |
-0,09 |
0 |
-0,15 |
-0,15* |
0,83** |
0,11 |
1 |
Aggressive attitude
The results showed that the family demographic and the kid’s age did not significantly account for the variation in the aggressive behaviour of kids, F(4180) = 2,01,p = 0,12. Step 2’s sleep CSHQ subscale values contributed an extra 16 % to the explanation of the variation in aggressive attitude, FΔ(4990) = 11,01,p < 0,001. The aggressive attitude was more prevalent in kids who had higher sleep issues linked to “sleep onset, duration, and increased night waking.” 17 % of the overall variations in kids aggressive attitudes was explained by the overall system, F(9190) = 6,98,p < 0,001. Outcomes for the HMR system’s aggressive attitude are synopsized and presented in Table 6.
|
Table 6. HMR system aggressive attitude outcomes |
||||||||||
|
|
Step 1 |
Step 2 |
||||||||
|
kid’s age |
Income |
Education |
nationality |
STR |
SD |
NW |
DSs |
MW |
||
|
Aggressive Attitude |
B |
0,17 |
-0,32 |
-0,18 |
0 |
-0,59 |
4,92 |
4,02 |
0,48 |
0,47 |
|
SE |
0,15 |
0,19 |
0,23 |
1,12 |
0,68 |
1,29 |
1,34 |
1,22 |
0,91 |
|
|
β |
0,11 |
-0,14 |
-0,11 |
0 |
-0,08 |
0,24*** |
0,20*** |
0,09 |
0,06 |
|
|
R2=0,04 |
∆R2=0,19*** |
|||||||||
Symptoms of depression and stress
Kids symptoms of stress and depression varied significantly, and step 1’s family demographic and ages of the kids helped clarify this, F(4180) = 3,18,p < 0,05. In step 2, the results of the CSHQ subscales significantly further clarified 13 % of the variation in the symptoms of depression and stress, FΔ(4990) = 8,68,p < 0,001. Symptoms of stress and depression were more prevalent in kids who had higher sleep issues connected to “STR, increased NW, and challenges linked to MW.” 18 % of the total variance in kids absorbing behaviour was explained by the overall system, F(9190) = 6,39,p < 0,001. Outcomes for HMR system stress and depression are synopsized and presented in table 7.
|
Table 7. HMR system stress and depression outcomes |
||||||||||
|
|
|
Step 1 |
Step 2 |
|||||||
|
Kid’s Age |
Income |
Education |
Nationality |
STR |
SD |
NW |
Sds |
MW |
||
|
Stress and Depressed |
B |
0,08 |
-0,06 |
-0,07 |
-0,38 |
0,25 |
0,38 |
1,04 |
0,48 |
0,93 |
|
SE |
0,09 |
0,12 |
0,06 |
0,09 |
0,08 |
0,13 |
0,14 |
0,34 |
0,45 |
|
|
β |
0,11 |
-0,6 |
-0,55 |
-0,35 |
0,80 |
0,08* |
0,18* |
0,13 |
0,21* |
|
|
R2=0,03 |
∆R2=0,15*** |
|||||||||
Concentration issues
Kids concentration issues varied significantly, which was significantly explained by the ages of the kids and the family demographic indicated in step 1, R2 = 0,04, F (4180) = 2,69, p < 0,05. Step 2 revealed that CSHQ subscale ratings explained 15 % of the variation in concentration issues, FΔ(4990) = 7,21,p < 0,001. Particularly, kids who experienced significant challenges with “sleep initiation, sleep length, and sleep-disordered breathing experienced significant concentration issues.” 15 % of the variation in kids concentration issues was explained overall by the system, F(9190) = 5,31,p < 0,001. Outcomes for HMR system concentration issues are synopsized and presented in table 8.
|
Table 8. HMR system concentration issues |
||||||||||
|
|
|
Step 1 |
Step 2 |
|||||||
|
|
|
Kid’s Age |
Income |
Education |
Nationality |
STR |
SD |
NW |
SD |
MW |
|
Concentration issues |
B |
0,12 |
-0,14 |
-0,07 |
-0,18 |
0,05 |
1,25 |
0,35 |
0,82 |
-0,05 |
|
SE |
0,05 |
0,09 |
0,08 |
0,28 |
0,18 |
0,26 |
0,34 |
0,38 |
0,27 |
|
|
|
0,16 |
-0,08* |
-0,11 |
-0,08 |
0,05 |
0,14*** |
0,15 |
0,24* |
-0,05 |
|
|
R2=0,06 |
∆R2=0,14*** |
|||||||||
CONCLUSION
In this research, we provided a brand-new CSHQ measurement tool that identifies the sleep problems seen in 4 to 5 years old kids who have ASD, exhibits a good fit with the data collected, and shows a distinct pattern of connections to aggression, stress, symptoms of depression, and concentration issues. In addition, the system presents good fit to observed information, and it shows a good fit to observed data information, and there are limitations also. First, because our sample size was insufficient to perform different analyses in both training and confirmation sets, we failed to validate the developed measurement framework with a separate sample in the present research. Replication is necessary. Second, we lacked a control group of typically developing children aims to decide if the CSHQ exhibits the same level of factorial reliability as a sample of young children. However, prior research suggests that sleep issues in kids who have ASD have a similar developmental pattern to kids who are usually developing, with essentially identical symptoms in kids who have ASD but more frequent or severe symptoms compared to peers their same age who are normally developing. Therefore, one may anticipate that the structure of the variables would be comparable, but this would need to be established experimentally in future studies.
REFERENCES
1. MacDonald LL, Gray L, Loring W, Wyatt A, Bonnet K, Schlund D, Gaston ML, Malow BA. A community-based sleep educational intervention for children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2021;81:101719. https://doi.org/10.1016/j.rasd.2020.101719
2. Lindor E, Sivaratnam C, May T, Stefanac N, Howells K, Rinehart N. Problem behavior in autism spectrum disorder: considering core symptom severity and accompanying sleep disturbance. Frontiers in Psychiatry. 2019;10:487. https://doi.org/10.3389/fpsyt.2019.00487
3. Galli J, Loi E, Visconti LM, Mattei P, Eusebi A, Calza S, Fazzi E, ASD Collaborative Group. Sleep disturbances in children affected by autism spectrum disorder. Frontiers in Psychiatry. 2022;13:736696. https://doi.org/10.3389/fpsyt.2022.736696
4. Kamal Nor N, Ghozali AH, Ismail J. Prevalence of overweight and obesity among children and adolescents with autism spectrum disorder and associated risk factors. Frontiers in Pediatrics. 2019;7:38. https://doi.org/10.3389/fped.2019.00038
5. Leader G, Glynn C, Kirkpatrick B, Chen JL, O’Súilleabháin PS, Mannion A. Familial sleep and autism spectrum disorder: a pilot actigraphy study of sleep quality, quality of life and psychological distress. Irish Journal of Psychological Medicine. 2022;39(3):261-71. https://doi.org/10.1017/ipm.2021.24
6. Ann Abraham D, Narasimhan U, Christy S, Muhasaparur Ganesan R. Effect of L-Carnosine as adjunctive therapy in the management of children with autism spectrum disorder: a randomized controlled study. Amino Acids. 2020;52(11):1521-8. https://doi.org/10.1007/s00726-020-02909-1
7. Benson S, Bender AM, Wickenheiser H, Naylor A, Clarke M, Samuels CH, Werthner P. Differences in sleep patterns, sleepiness, and physical activity levels between young adults with autism spectrum disorder and typically developing controls. Developmental Neurorehabilitation. 2019;22(3):164-73. https://doi.org/10.1080/17518423.2018.1501777
8. Johnson CR, Smith T, DeMand A, Lecavalier L, Evans V, Gurka M, Swiezy N, Bearss K, Scahill L. Exploring sleep quality of young children with autism spectrum disorder and disruptive behaviors. Sleep Medicine. 2018;44:61-6. https://doi.org/10.1016/j.sleep.2018.01.008
9. Tyagi V, Juneja M, Jain R. Sleep problems and their correlates in children with autism spectrum disorder: an Indian study. Journal of Autism and Developmental Disorders. 2019;49:1169-81. https://doi.org/10.1007/s10803-018-3820-6
10. Xiong M, Li F, Liu Z, Xie X, Shen H, Li W, Wei L, He R. Efficacy of melatonin for insomnia in children with autism spectrum disorder: a meta-analysis. Neuropediatrics. 2023;54(3):167-73. https://doi.org/10.1055/s-0043-1761437
11. Inthikoot N, Chonchaiya W. Sleep problems in children with autism spectrum disorder and typical development. Pediatrics International. 2021;63(6):649-57. https://doi.org/10.1111/ped.14496
12. Yavuz-Kodat E, Reynaud E, Geoffray MM, Limousin N, Franco P, Bourgin P, Schroder CM. Validity of actigraphy compared to polysomnography for sleep assessment in children with autism spectrum disorder. Frontiers in Psychiatry. 2019;10:551. https://doi.org/10.3389/fpsyt.2019.00551
13. Malow BA, Findling RL, Schroder CM, Maras A, Breddy J, Nir T, Zisapel N, Gringras P. Sleep, growth, and puberty after 2 years of prolonged-release melatonin in children with autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2021;60(2):252-61. https://doi.org/10.1016/j.jaac.2019.12.007
14. Bhadra U, Thakkar N, Das P, Bhadra MP. Evolution of circadian rhythms: from bacteria to human. Sleep Medicine. 2017;35:49-61. https://doi.org/10.1016/j.sleep.2017.04.008
15. Kang YQ, Song XR, Wang GF, Su YY, Li PY, Zhang X. Sleep problems influence emotional/behavioral symptoms and repetitive behavior in preschool-aged children with autism spectrum disorder in the unique social context of China. Frontiers in Psychiatry. 2020;11:273. https://doi.org/10.3389/fpsyt.2020.00273
FINANCING
None.
CONFLICT OF INTEREST
None.
AUTHORSHIP CONTRIBUTION
Conceptualization: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Data curation: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Formal analysis: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Research: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Methodology: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Project management: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Resources: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Software: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Supervision: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Validation: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Display: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Drafting - original draft: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
Writing - proofreading and editing: Bharat Bhushan, Sameer Rastogi, Vijay Jagdish Upadhye, Banani Jena, Anoop Dev, Sunil Lawand.
APPENDIX
|
Autism spectrum disorder = ASD |
Tucker–Lewis Index = TI |
|
Children’s Sleep Habits Questionnaire = CSHQ |
Cronbach’s alpha = CA |
|
Exploratory factor analysis = EFA |
Sleep-time routine = STR |
|
Confirmatory factor analysis = CFA |
Sleep duration = SD |
|
Merrill-Palmer-Revised Scales of Development = M-P-R |
Night Wakes = NW |
|
Daytime Sleepiness = DS |
Bedtime Resistance = BR |
|
Autism Diagnostic Observation Schedule = ADOS |
Sleeping disordered = SDs |
|
Root mean square error of approximation = RMSEA |
Morning Wakes = MW |
|
Morning waking = MW |
Hierarchical multiple regression= HMR |
|
Child Behavior Checklist = CBC |
|